In collaboration with the University of Sydney’s Biomedical Engineering Faculty, Vestech Medical developed an emergency ventilator in response to the emerging COVID-19 pandemic. Despite time pressure and supply issues, the team at Vestech Medical designed, manufactured, and tested the ventilator with the technical expertise of collaborators to meet the TGA’s specifications. Under Vestech Medical’s project management, the team successfully received TGA clearance for supply under COVID-19 Emergency Exemption Legislation within 10 months from commencement.
ABOUT VESTECH MEDICAL
OUR QUALITY POLICY

CASE STUDIES
CoVida FC100 VENTILATOR with USYD

NUMEDICO TECHNOLOGIES

Numedico Technologies is utilising Vestech Medical to develop their patented and innovative syringe designs, gain regulatory approval, and act as the legal manufacturer. Our client has made full use of Vestech Medical’s expertise in the areas of new product introduction (NPI), prototyping, feasibility design de-risking, patent strategy, project management, quality management systems, regulatory know how and manufacturing. Tasked with sourcing long-life, high cavitation, injection mold tooling whilst building a fully automated syringe assembly line within a cleanroom environment, Vestech Medical is future-proofing the manufacturing line for Numedico Technologies. With Vestech Medical’s expertise, Numedico Technologies is enhancing their chances of design and regulatory submission success.
ResusRight
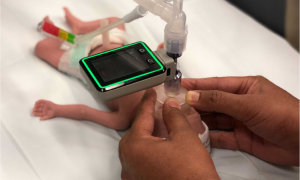
ResusRight is an Australian-based MedTech startup developed a resuscitation monitor to advance the gold standard of clinical training and practice of neonatal resuscitation. With clinical and engineering input and knowledge, ResusRight had all the tools needed to develop a compelling and life saving device but needed help on the compliance and regulatory aspects. Vestech Medical stepped in and guided them along this path, a path they took ownership of and excelled in travelling.
LACTAMO

Inspired by the clinical and human need, Lactamo’s founder, Etta, knew she had to create a solution to assist with common breastfeeding problems. Vestech Medical provided assistance in prototyping and problem solving to guide Lactamo through the quality and regulatory steps required for success. Lactamo is now on the market and available, helping women with common breastfeeding problems including oversupply, undersupply, blocked ducts, mastitis and engorgement.
LENEXA MEDICAL

Pressure injuries (or bed sores) are a mostly preventable but devasting health problem, especially with the elderly in long-stay wards and aged-care facilities. Risk of pressure injury is dependent on patient resting position and time. Lenexa Medical as developed a world-first fabric-based sensor technology paired with AI software that monitors patient position and pressures in real-time to enable personalised pressure injury (or ‘bed sore’) care. Vestech Medical provided Quality Management System, Regulatory Affairs and Technical support during the development project, leading to the successful inclusion of the LenexaCARE system onto the ARTG.
Saving this place for you

CEO Dr Greg Roger on Why he started Vestech Medical
My experience in medical device development has shown me first-hand how many ways it can go wrong – how unlikely it is that an inventor, scientist, or doctor is able to anticipate all the steps required to achieve success and how to put those steps together. Over the years, the team at Vestech Medical has not just studied this but have actually done it by successfully launching medical devices on to the market in the United States, Europe, and Asia-Pacific. Our expertise comes not only from deep knowledge, but also from having walked the walk.
The unique aspect of Vestech Medical is the broad range of skillsets the team has in manufacturing expertise, research expertise, and having been inventors ourselves. We have experienced the joys and the heights of invention and realisation of a new device and the lows of the practical step-by-step challenges that occur every day for years, not months, in the process of doing that.
I’ve seen how much harm can be done to the inventor, friends, and family as the process unravels, rather than evolves. Preventing that harm is the key reason I wanted to start Vestech Medical. I want to harvest all the experience that the team has gained over the years and decades to help people to make the right decisions at the right time, understand what’s going to be in front of them, and understand what they need to do. But most of all understand where the hell they’re going. If you don’t know where you’re going, you’re not going to get there.
Australia is renowned for a nation of great innovation, but we lag in commercialisation. Everybody knows it, we’re just not good at it. Wanting to make a difference in that space has taken me to federal government, state government, advisory roles, and starting two companies (Vestech Medical and ASDM, now called Allegra Orthopaedics). I do this because I really hope to see an industry in Australia. It’s only with a deep industry – lots and lots of companies competing, working together, engaging vendors with specific expertise – that we can all thrive. So that’s really been my aim over time, to see our industry grow. At Vestech Medical we’re helping that by helping other people to start new companies, employ people, drive innovation, and hopefully realise their dreams.

